Thesis overview
Evaxion (EVAX) aspires to use artificial intelligence (AI) to design vaccines for cancer immunotherapy, as well as for infectious diseases. EVAX claims being more advanced than competition because it has been constantly developing and improving its models for 15 years. Additionally, EVAX has in-house capability to validate novel targets identified by its AI models in state-of-the-art animal models.
Although I can't claim to have a deep understanding of Evaxion's AI technology there are good reasons to be bullish about EVAX, including multiple collaborations (including a collaboration with MSD, which is also a 15% shareholder), as well as proof-of-concept (POC) preclinical and clinical data in both infectious diseases and oncology. Additionally, EVAX has recently raised sufficient cash to last through 2024, during which time EVAX aims to report achievement of several additional milestones (more POC data, potentially new partnerships, and/or progress with existing partnership) that could boost the stock price. The major risk to the thesis is the need for a lot more cash to progress with the pipeline. Nevertheless, I believe EVAX has a good chance of establishing more partnerships and good news during 2024 could help EVAX raise cash from a stronger position and/or non-dilutively.
Overview of Evaxion's strategy
EVAX aims to use its AI platform for the following purposes;
- To quickly and efficiently identify appropriate antigenic epitope targets that are predicted to elicit protective B-cell and/or T-cell responses. Selected epitopes can then be incorporated into vaccine constructs. Evaxion's model can be used for vaccine development both in infectious diseases and oncology.
- To predict which patients will respond to treatment with immune checkpoint inhibitors. The goal is to both improve patient outcomes (by guiding personalised treatment algorithms and timelier use of the treatment most likely to work) and to save costs (by avoiding use of expensive treatments that are unlikely to work).
Importantly for an early-stage biotech, EVAX's business model is currently based on partnerships, with already 3 partnerships established, including a partnership with MSD validating big pharma interest. Notably, EVAX aspires to cover all 2014 operating expenses through business transactions. EVAX still has several other partner-ready assets.
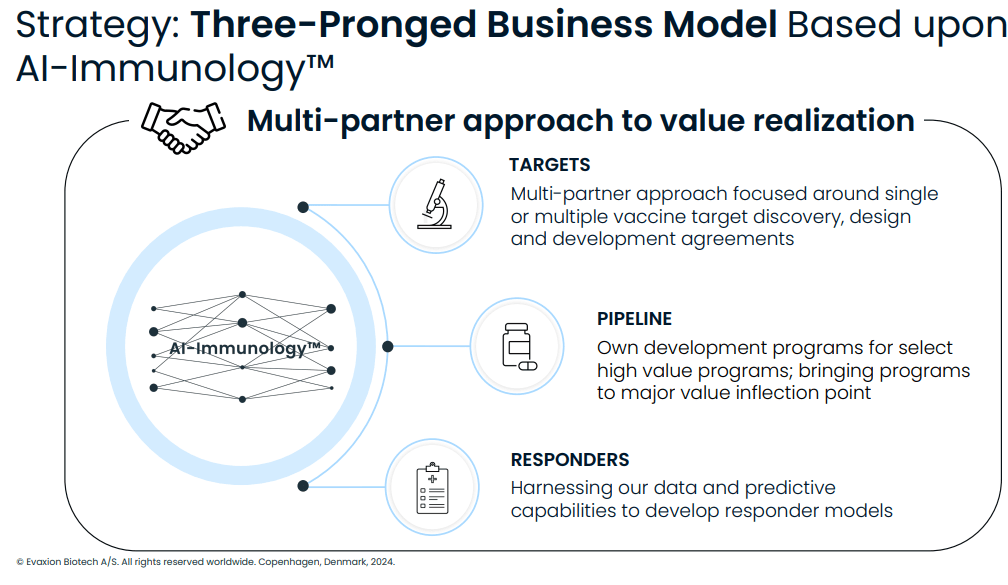
Evaxion's business model (Evaxion's R&D day, March 19, 2024)
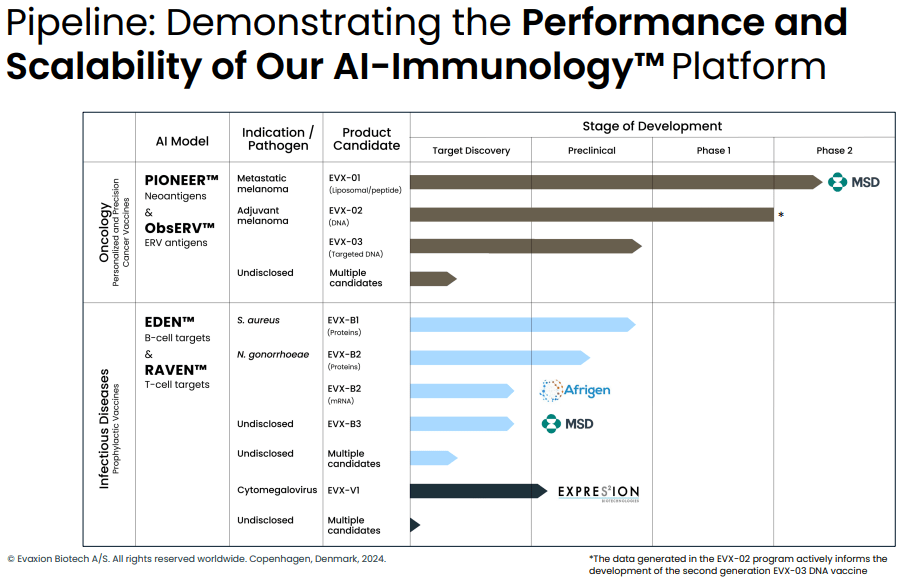
Evaxion's pipeline and partnerships (Evaxion's R&D day, March 19, 2024)
Overview of Evaxion's AI models
Evaxion's AI models are summarized below;
- EDEN; AI model to discover protective B-cell antigens. These are aimed to induce a humoral immune response, i.e. induction of protective antibodies. The model is currently used for development of infectious diseases vaccines, and seems to mainly target high-priority bacterial pathogens for which vaccines are not yet available.
- RAVEN: AI model to discover protective T-cell antigens. These are aimed to elicit a cellular immune response, i.e. induction of helper T-cells that help B-cells produce antibodies and/or T-cells that kill infected cells. The model can be used in combination with EDEN model to create vaccines that combine B-cell and T-cell epitopes.
- PIONEER: AI model for designing personalized neoantigen cancer vaccines.
- ObsERV: AI model for designing cancer vaccines targeting endogenous retroviruses (ERV).
- AI-DEEP: AI model aiming to accurately predict which patients will respond to immune checkpoint inhibitors (with potential application to other treatments as well).
The vaccine models generally work in 3 steps: (1) identification of most promising antigens based on disease decoding, (2) selection of antigens most likely to induce a protective immune response, (3) vaccine design. This is followed by in-house preclinical validation in animal models. Below I discuss each AI model separately. For a more detailed presentation I refer interested readers to the recent R&D day presentation.
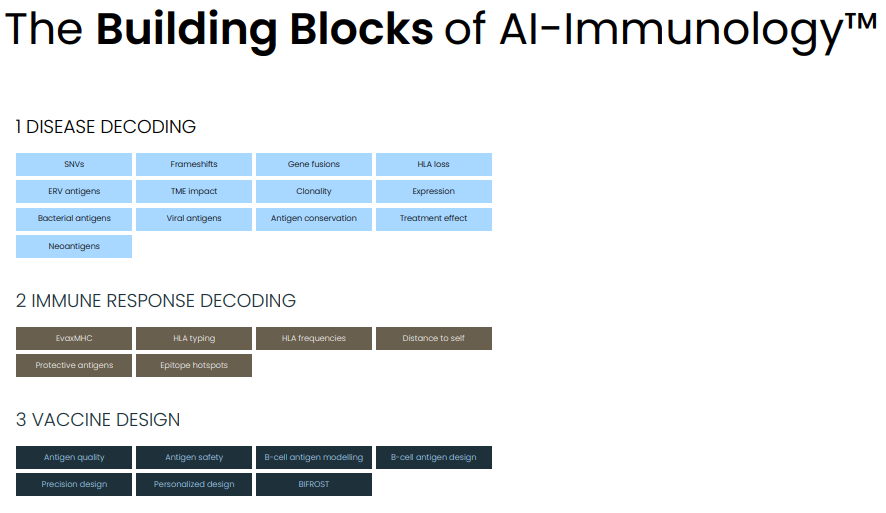
Steps of the AI models (Evaxion's R&D day, March 19, 2024)
EDEN (protective B-cell antigens)
EVAX claims that EDEN is a best-in-class model for assessing protectiveness of B-cell antigens. The platform is based on 3 steps:
- Step 1: Ranking of pathogen proteins to find most protective B-cell antigen.
- Step 2: Validation of the protectiveness of identified antigens in animal models.
- Step 3: Design and testing of vaccine based on identified antigens.
Notably, in their R&D day EVAX compares their technology to traditional reverse vaccinology which has been used by Novartis and Intercell to identify protective antigens for Group A Streptococcus. Impressively, EVAX's AI technology identified within 24 hours all highly protective antigens that had been identified by reverse vaccinology (years of work), as well as multiple completely novel protective antigens (see image below).
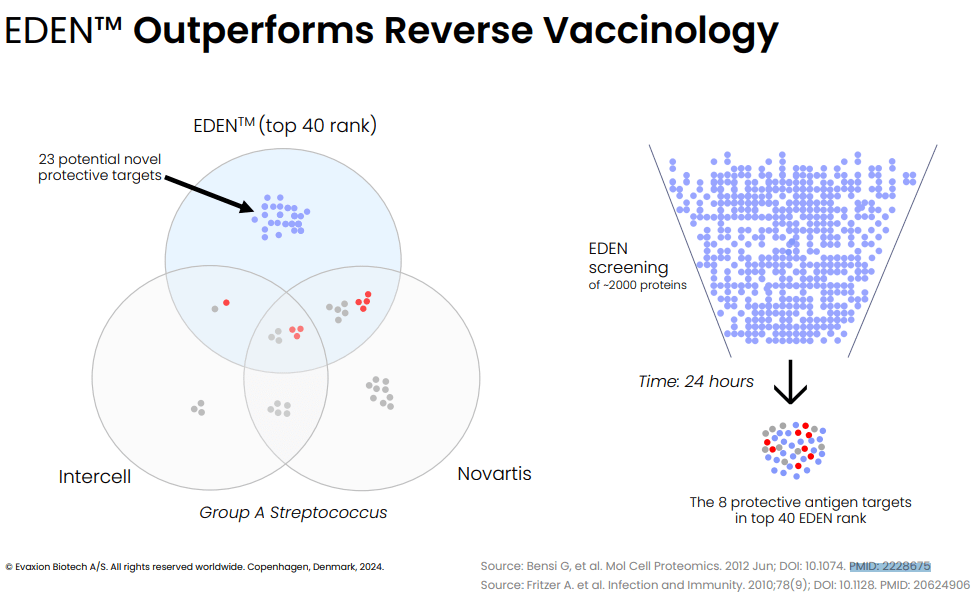
EDEN vs reverse vaccinology. Each small circle represents identified antigens. The red ones represent highly protective antigens. (Evaxion's R&D day, March 19, 2024)
EVAX aims to use this platform (in combination with RAVEN described below) to develop vaccines against high-priority pathogens, for which there are currently no available vaccines, including N. gonorrhea, A. baumannii, K. pneumoniae and S. aureus. All these are high-burden pathogens (affecting a large number of patients and associated with considerable morbidity/mortality) and characterized by increasing resistance (even pandrug-resistance in the case of A. baumannii) to antibiotics. All are included in the CDC's list of pathogens that represent a threat.
Using EDEN, EVAX has identified the two most promising antigenic targets against N. gonorrhea, which EVAX has combined in a single vaccine ((EVX-B2)). Notably, the identified antigens represent a new class of antigens , present for targeting only during cell division, highlighting the capabilities of the AI model to identify completely novel target antigens. EVAX has shown that:
- EVX-B2 elicits a strong protective response
- Serum from EVX-B2-treated mice has bactericidal efficacy against a broad panel of 50 clinically relevant N. gonorrhea strains
- EVX-B2 shows stronger protection than lead clinical candidate.
More details about EVX-B2 can be found in the relevant publication in mBio. EVX-B2 has been partnered with Afrigen Biologics.
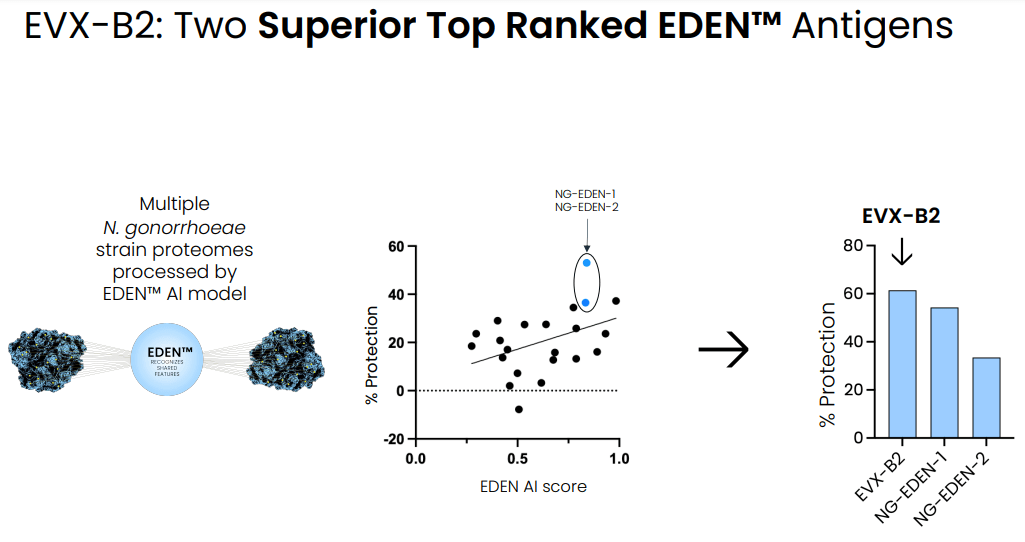
Identification of 2 highly protective antigens combined in a single vaccine antigen against N. gonorrhea (Evaxion's R&D day, March 19, 2024)
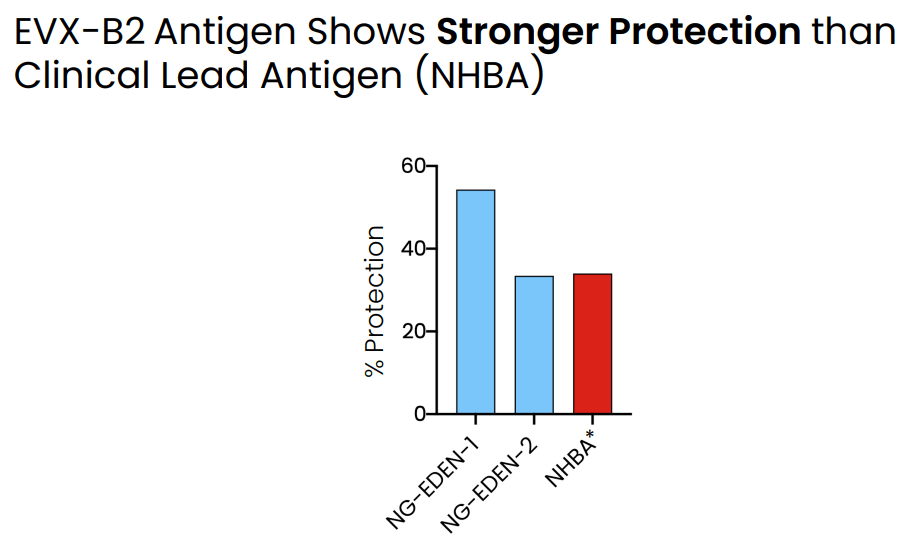
EVX-B2 shows stronger protection than lead clinical candidate being developed by GSK (Evaxion's R&D day, March 19, 2024)
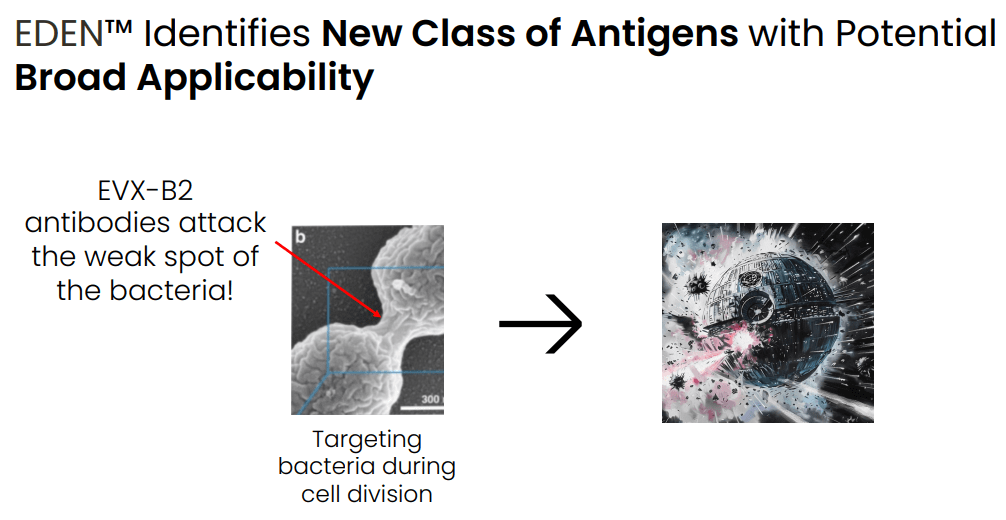
POC that EVAX's AI models can identify completely novel antigenic targets (Evaxion R&D day, March 19, 2024)
In summary, EVAX has provided convincing POC data illustrating the competitive advantages of its AI model:
- Fast (target identification within 24 hours).
- Potential to identify completely novel antigenic targets.
- Potential to identify antigenic targets that are more protective than targets identified by competing methods.
In addition to EVX-B2, EVAX is also developing EVX-B1 targeting S. aureus, as well as multiple undisclosed candidates in the target discovery stage. Notably, EVAX has presented POC preclinical data in animal models for skin infection and sepsis for EVX-B1, showing durable (at least 12 months) antibody responses and protection against development of disease, as well as elimination of the infection.
RAVEN (protective T-cell antigens)
RAVEN is a model for assessing protectiveness of T-cell antigens. There are 3 ways to use the platform:
- Design of stand-alone T-cell vaccine. T-cell vaccines are not as effective as B-cell vaccines but have the advantage that can be produced extremely fast, which could prove to be useful in a future pandemic in need of a new vaccine.
- T-cell antigen priming followed by B-cell antigen boosting, to improve quality of the immune response.
- Integrated T-cell/B-cell antigen approach. RAVEN and EDEN models can be combined to design vaccines that combine B-cell and T-cell epitopes and have the potential to induce a broader antibody response.
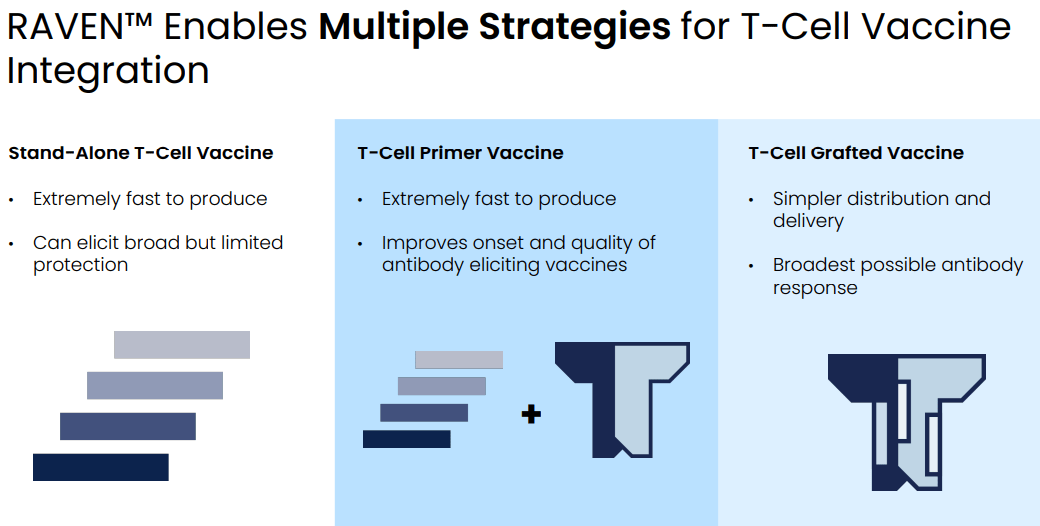
Strategies for T-cell vaccines (Evaxion R&D day, March 19, 2024)
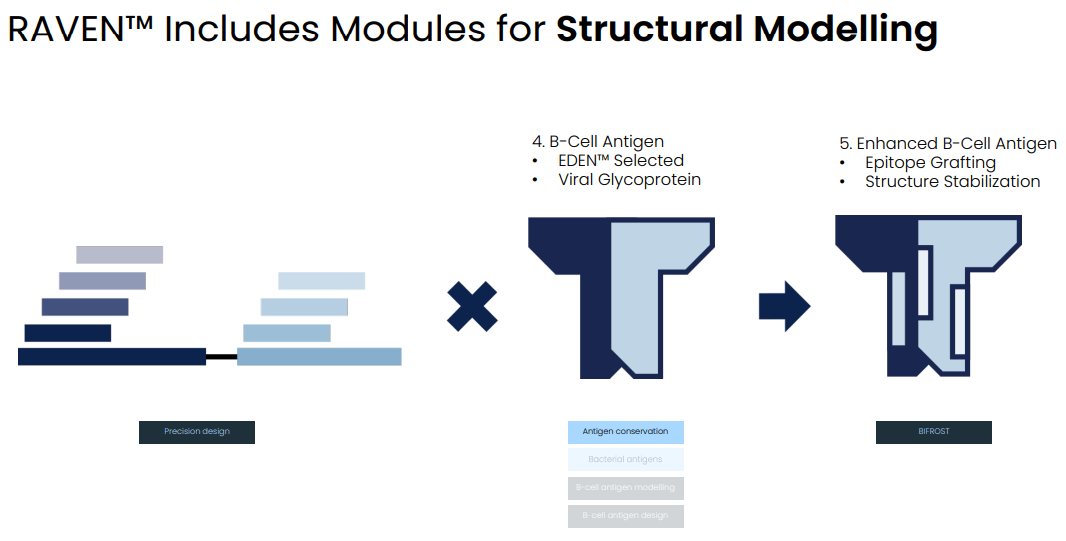
RAVEN can be combined with EDEN to develop vaccine antigens containing both B-cell and T-cell epitopes (Evaxion's R&D day, March 19, 2024)
Based on the combination of RAVEN and EDEN AI models EVAX has developed two candidates: EVX-B3 (targeting an undisclosed bacterium in collaboration with MSD) and EVX-V1 (targeting CMV, in collaboration with ExpreS2ion).
PIONEER (neoantigen platform)
Mutations in cancer cells can result in expression of new antigens (neoantigens) present in cancer cells but not in healthy cells. Neoantigen-based vaccines aim to exploit this. Notably, the set of neoantigens differs considerably between patients. PIONEER AI model aims to identify patient-specific cancer targets for the development of personalized cancer vaccines. It works in the following steps:
- Step 1: Identify neoantigens based on patient-specific tumor mutations
- Step 2: Identify neoantigens that will bind MHC (i.e. neoantigens that are predicted to be presented in the surface of cancer cells)
- Step 3: Identify neoantigens that will elicit a T-cell response
- Step 4: Identify a set of neoantigens that will elicit a T-cell response against all tumor cells. Even within the same tumor cancer cells are heterogeneous. Targeting a single neoantigen would just result in emergence of subpopulations of tumor cells that do not express the targeted neoantigen.
Notably, the personalized vaccine can be prepared within 6-8 weeks.
As with any cancer vaccine, a major hurdle is that tumors have a variety of protective mechanisms that allow tumors to evade/suppress the immune system within the tumor microenvironment. Hence, such vaccines are aimed to be used in combination with other immunotherapies, such as checkpoint inhibitors.
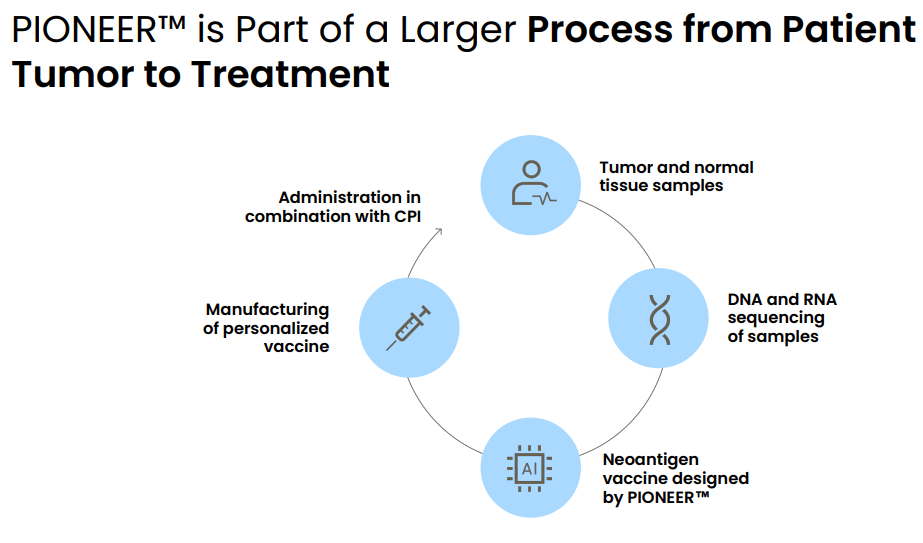
Process from patient tumor to treatment using PIONEER. This takes about 6-8 weeks. (Evaxion's R&D day, March 19, 2024)
Based on the PIONEER AI model EVAX has developed the following assets:
- EVX-01 (a peptide vaccine) being studied in metastatic melanoma
- EVX-02 (a DNA vaccine) which has been studied in the adjuvant setting (post-resection)
Additionally EVAX has multiple other undisclosed candidates in target discovery stage.
POC data in melanoma
Currently, EVAX has only two clinical stage assets, EVX-01 and EVX-02, both for melanoma. Both have completed early phase clinical studies.
In a phase 1 study in n=12 patients with stage IV metastatic melanoma the combination of EVX-01 with anti-PD1 (pembro or nivo) was associated with 67% ORR (CR: n = 2, PR: n = 6). Furthermore, neoantigen specific T cells were induced by the vaccine in all patients analyzed and this was dose dependent. Additionally, response to treatment was better in patients with better quality (higher PIONEER score) neoantigens (discussed in the next section). Finally, the treatment was safe and well-tolerated (safety and tolerability being the primary endpoint of the study).
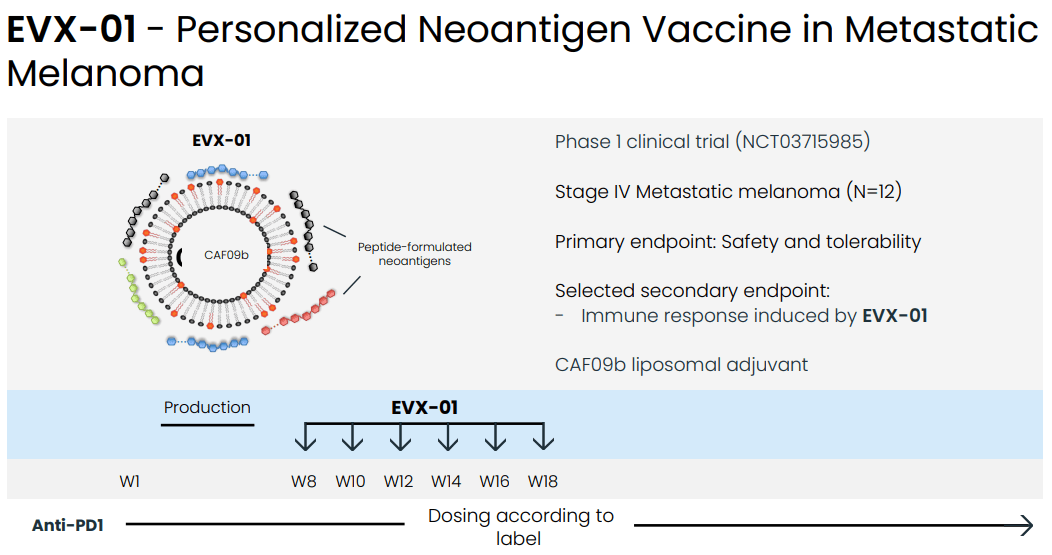
EVX-01 ph1 study design (Evaxion's R&D day, March 19, 2024)
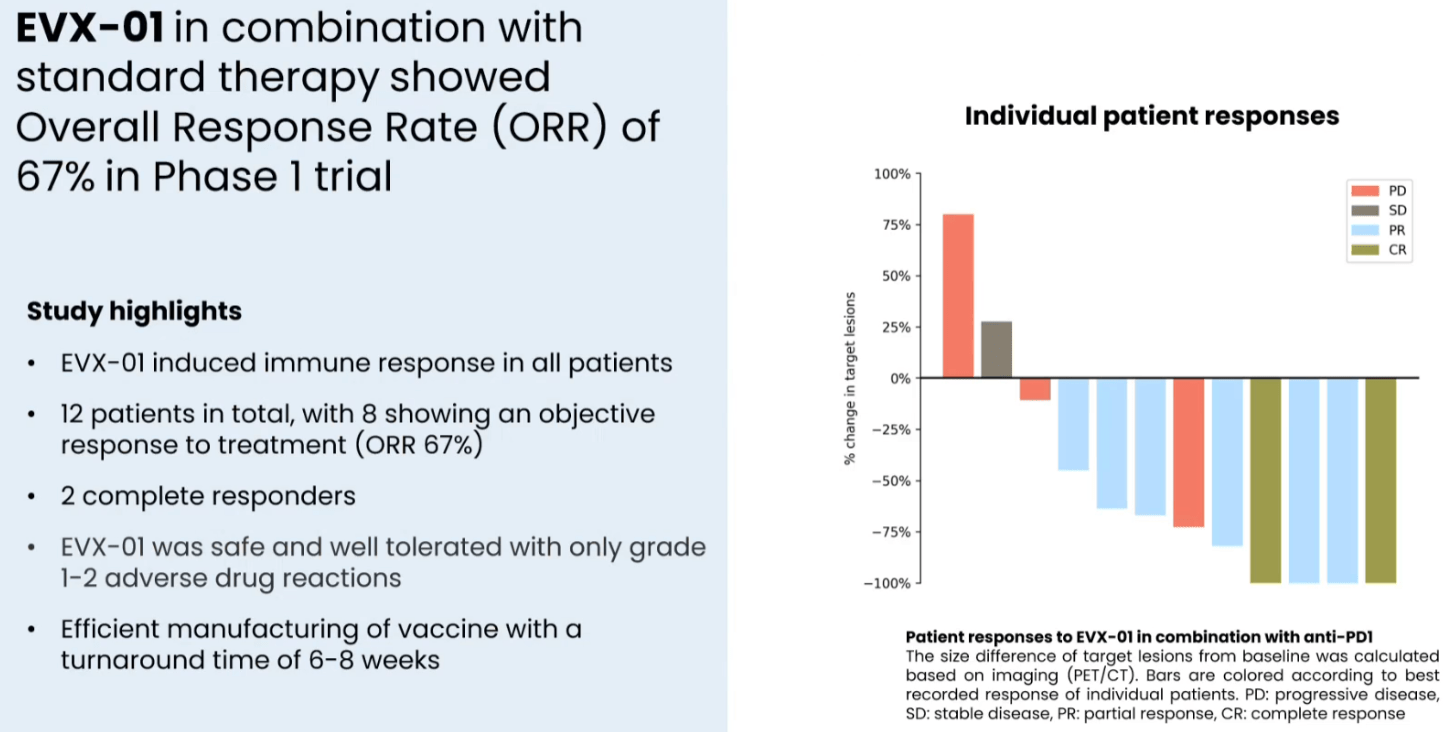
Efficacy of EVX-01 in combination with ICI in the phase 1 trial (Presentation of Latest Confirmatory EVX-01 Clinical Data with Key Opinion Leader Adnan Khattak)
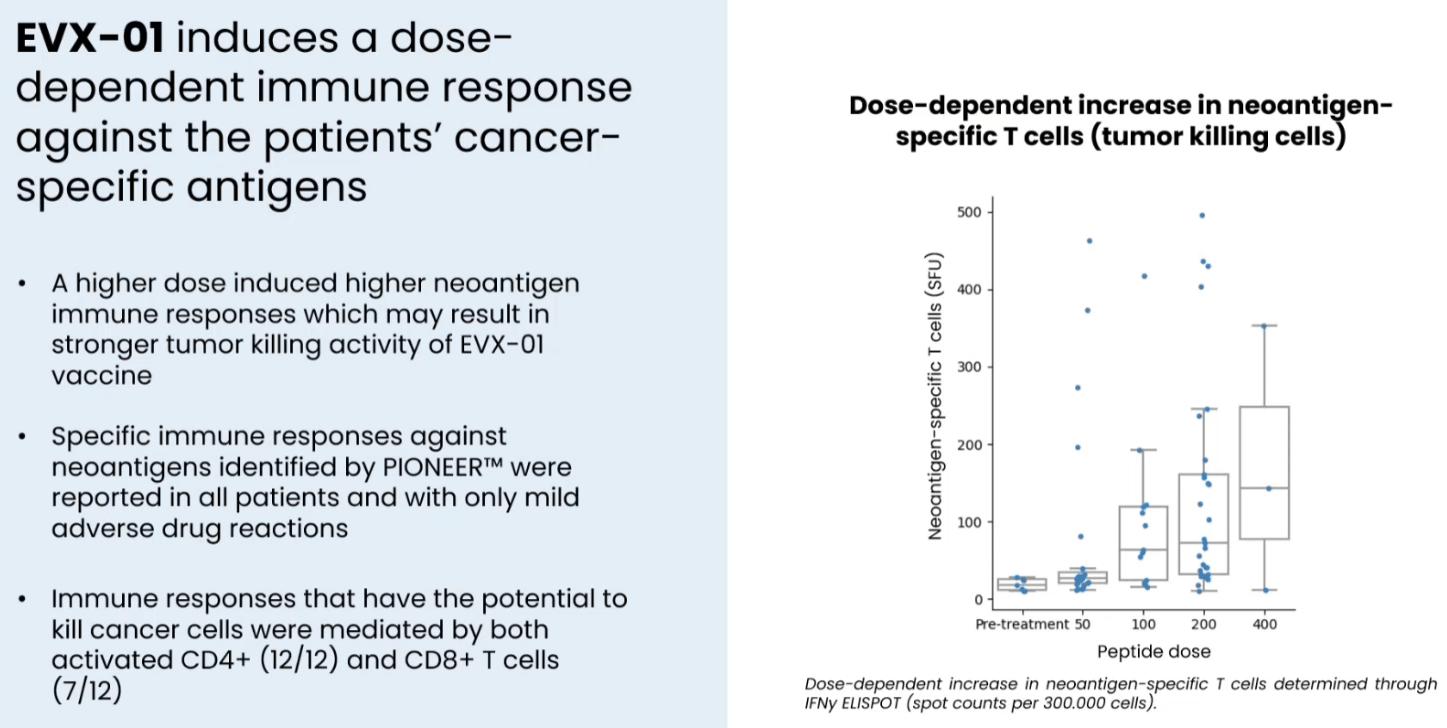
T-cell responses induced by EVX-01 in the phase 1 study (Presentation of Latest Confirmatory EVX-01 Clinical Data with Key Opinion Leader Adnan Khattak)
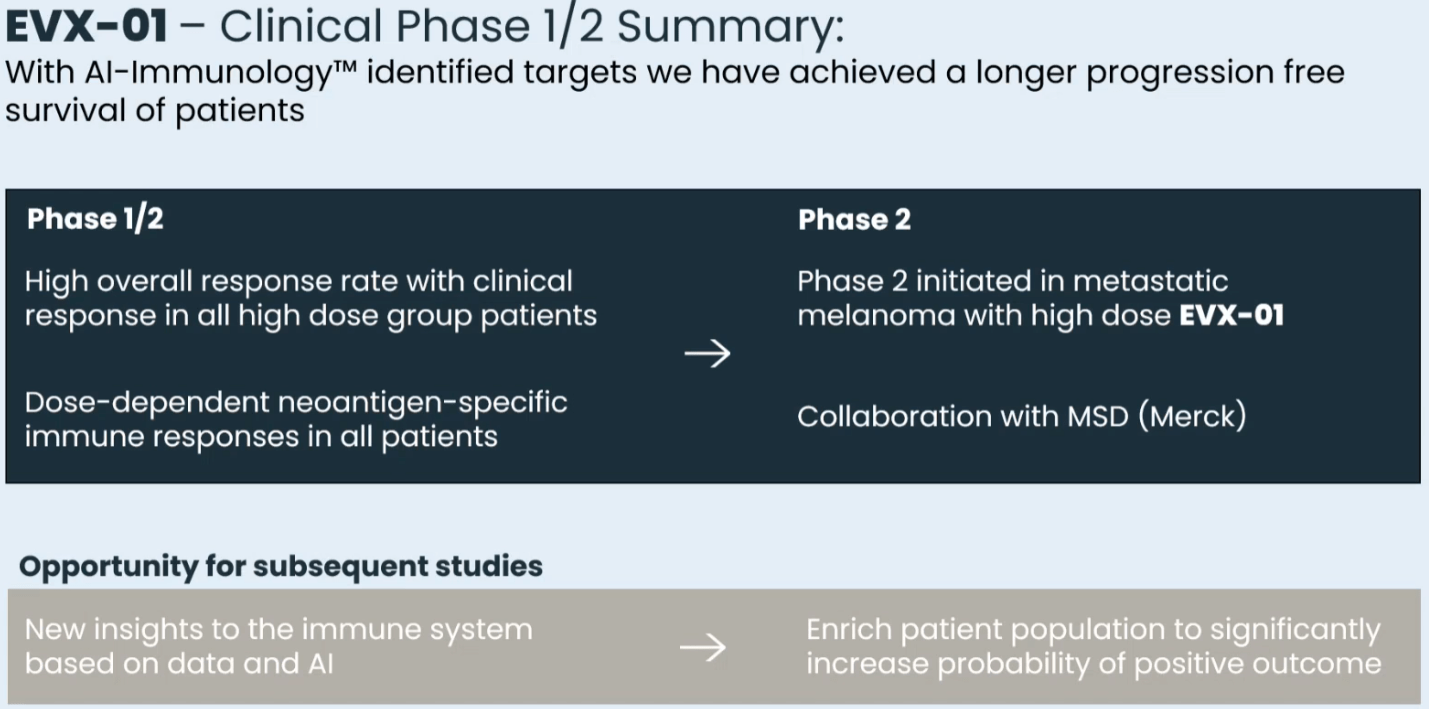
Clinical summary of EVX-01 ph 1/2 (Presentation of Latest Confirmatory EVX-01 Clinical Data with Key Opinion Leader Adnan Khattak)
EVX-01 is currently being evaluated in the higher dose in a phase 2 study in combination with pembrolizumab (which is provided by MSD under the collaboration agreement). EVX-01 has also been granted Fast Track designation by the FDA. Initial data from the first 5 patients were recently announced, confirming safety-tolerability and promising efficacy (see images below). Notable is the case of one patient who at the time of initiation of treatment with EVX-01, i.e. 12 weeks post-initiation of pembrolizumab, had progressive disease, in which case EVX-01 initiation was associated with reduction of tumor size. One-year readout is expected in Q3 2024, while final results are expected in 2025.
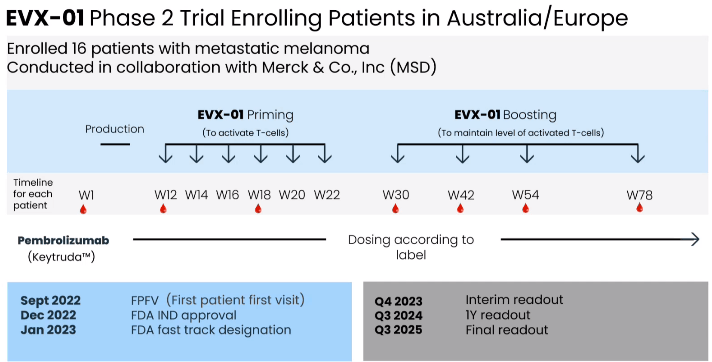
EVX-01 phase 2 study design and current progress (Presentation of Latest Confirmatory EVX-01 Clinical Data with Key Opinion Leader Adnan Khattak)
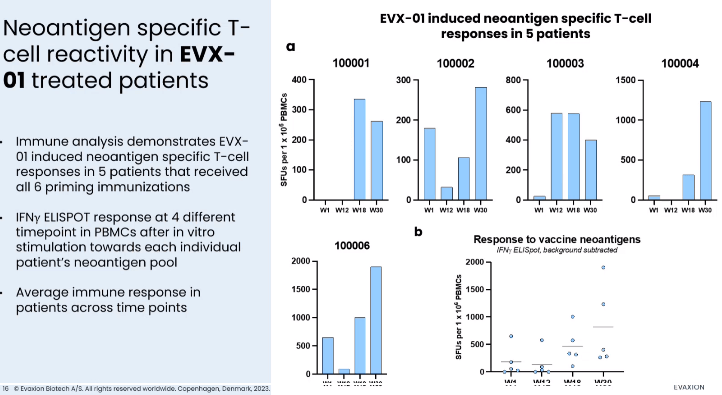
T-cell responses in the ongoing phase 2 study (Presentation of Latest Confirmatory EVX-01 Clinical Data with Key Opinion Leader Adnan Khattak)
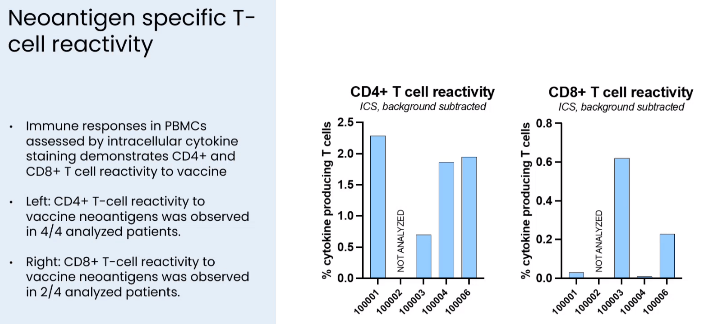
CD4+ vs CD8+ T-cell responses in the ongoing phase 2 trial (Presentation of Latest Confirmatory EVX-01 Clinical Data with Key Opinion Leader Adnan Khattak)
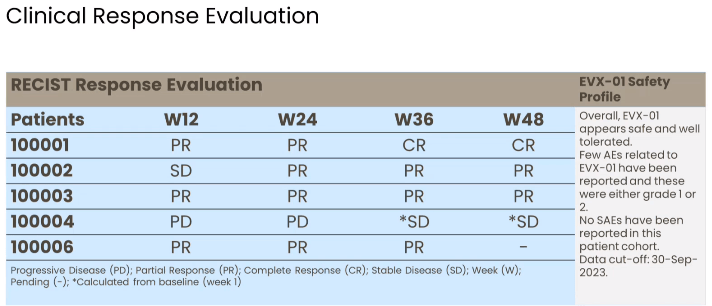
Tumor responses in the ongoing phase 2 study (Presentation of Latest Confirmatory EVX-01 Clinical Data with Key Opinion Leader Adnan Khattak)
CT scans of the patient who had progressive disease on pembrolizumab, but appear to have responded to initiation of EVX-01 (Presentation of Latest Confirmatory EVX-01 Clinical Data with Key Opinion Leader Adnan Khattak)
EVX-02 is a DNA personalized neoantigen-based vaccine which has been evaluated in the adjuvant setting (after resection of melanoma) in combination with anti-PD1 in n=10 patients. All 10 patients completed the eight planned immunizations and remained relapse-free at last assessment (9 of 10 were relapse-free at 12 months, 1 patients stopped the study prematurely due to a non-EVX-02 adverse event and remained relapse-free at last evaluation which was at 9 months). Treatment was well-tolerated and resulted in neoantigen T-cell responses (involving both CD4+ and CD8+ T cells) in all patients. Notably, being a DNA vaccine (vs EVX-01 which is a peptide vaccine), EVX-02 induced higher CD8+ T-cell responses (while EVX-01 induced higher CD4+ T-cell, and induced CD8+ response in only a subset of treated patients). EVAX believes a balance of both is necessary for optimal antitumor efficacy. EVAX does not plan to develop EVX-02 further but data generated informs the development of its 2nd generation DNA vaccine, EVX-03 (discussed in the next section).
- Sturdy and Durable: This OROPY wall mounted...
- Sleek Industrial Design: With its simple...
- Optimized Space Utilization: Expand your storage...
- Convenience at Your Fingertips: Hang your daily...
- Versatile Functionality: This multi-functional...
- 【Industrial Clothing Rack】 The clothing racks...
- 【Sturdy & Durable】 Our clothes racks are made...
- 【Height Adjustable】 The height of the lower...
- 【Multifunction Closet Rack】 Wall clothes rack...
- 【Multi-Scene Use】 Dimension: 115” W x87.5”...
- 【Safer Size/Style】: Whole sconces are UL...
- 【Outstanding Details】: Our high-quality black...
- 【NOTE】: Our bar lighting wall sconce include...
- 【Wide Application】: Vintage wall light...
- 【Tips】: As the tube bulb is a bit special, it...
Despite being promising, there are important limitations to above-discussed clinical data in melanoma. Given the lack of a control arm it is not possible to determine to what extent (if any) EVX-01/02 contributed to treatment efficacy. This is further complicated by the potential occurrence of pseudoprogression and/or delayed responses with immunotherapies. Nevertheless, elicited T-cell responses to neoantigens in all patients can be convincingly attributed to vaccination and above clinical studies have established in-human proof-of-concept data for Evaxion's AI Immunology platform, which alone is a major achievement.
ObsERV
As explained by EVAX: “ERVs are remnants of ancient viruses lying dormant in our genome. ERVs are often overexpressed in cancer but not in healthy tissue, making them visible to the immune system and hence promising targets for cancer vaccines. AI-Immunology™ is crucial in allowing the identification of therapeutically relevant ERV tumor antigens from genomic patient tumor data.” This is especially important for low tumor mutational burden cancers for which developing a personalized cancer vaccine based on neoantigens may not be possible. By combining missense mutations and ERV sources it is possible to design personalized cancer vaccines for more patients that it would be possible based on just missense mutations. In other words ERVs serve as an additional antigen source for personalized cancer vaccine design, which is particularly useful for patients with tumors without high-quality neoantigens (low PIONEER score).
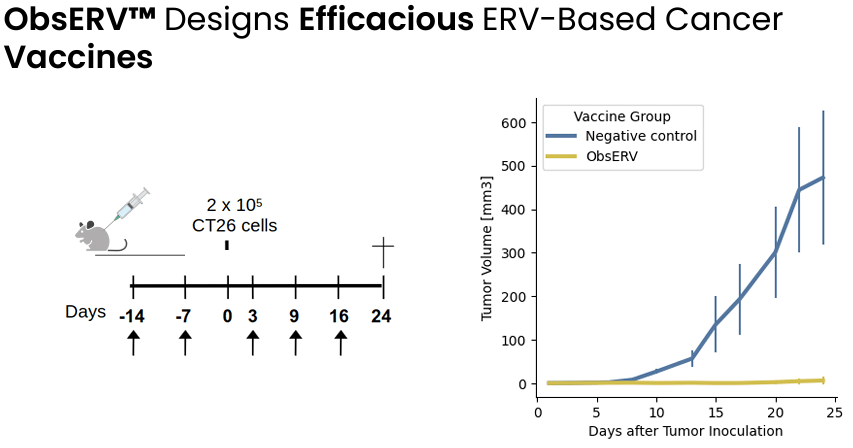
Preclinical proof-of-concept demonstrating prevention of tumor growth by ObsERV-designed EVR-based cancer vaccine (R&D Day on AI-Immunology™ March 19, 2024)
Based on the combination of PIONEER and ObsERV EVAX has developed EVX-03, a clinically-ready asset for the treatment of various cancers including non-small-cell-lung-cancer. EVX-03 is a DNA vaccine and thus expected to induced better CD8+ T-cell responses compared to EVX-01. Furthermore, EVX-03 is more sophisticated compared to EVX-02, including an additional payload and antigen selection combining PIONEER and ObsERV (see image below). EVX-03 also includes a CCL19 moiety, an antigen-presenting cell binding molecule, which is expected to considerably improve T-cell responses and anti-tumor efficacy. EVAX is “actively seeking partnership opportunities to further advance the development of the EVX-03 vaccine candidate”.
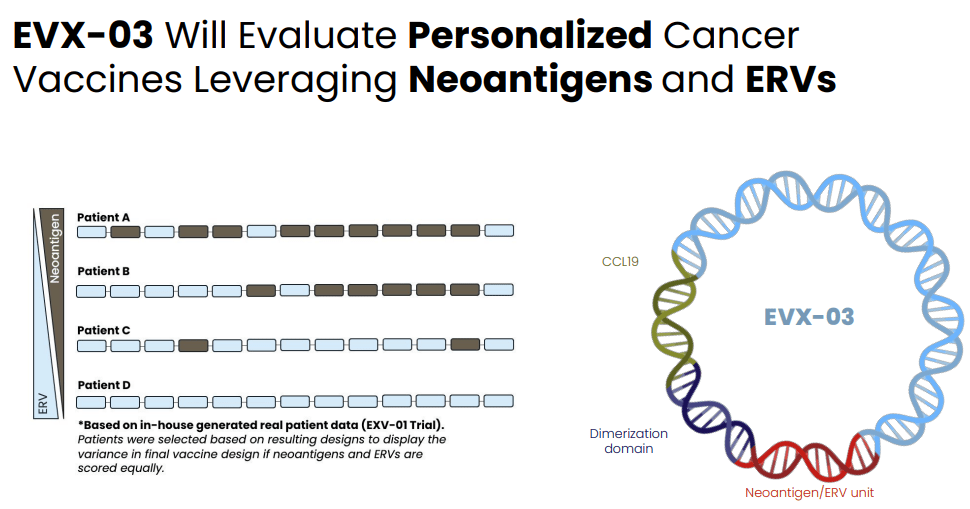
EVX-03 is a personalized DNA vaccine based on neoantigens and/or ERV antigens depending on the tumor characteristics of each patient (Evaxion's R&D day, March 19, 2024)
AI-DEEP
Despite considerable advances, immune checkpoint inhibitors work in only a subset of patients. However, it is not yet possible to reliably predict which patients will and which will not respond to treatment. Using an ineffective treatment has the following implications: (1) Patients not only have no benefit from the treatment, but are also exposed to treatment-related adverse effects, and lose precious time in search for an effective treatment. (2) Using ineffective therapies also results in considerable costs for healthcare systems without any benefit to the patient. Notably, the global immune checkpoint inhibitors market size is expected to reach about $150 billion by 2030. EVAX aspires to use its AI-DEEP model to accurately predict which patients will respond to immune checkpoint inhibitors (and potentially other treatments).
Evaxion's prediction model has been developed based on n=937 patients with a variety of tumors being treated with an immune checkpoint inhibitor. Notably, the model can predict with very high precision 28% of the non-responders. In other words, by using the model about a third of the patients that are not going to respond to treatment with an immune checkpoint inhibitor can be identified, and thus spared from being exposed to an ineffective treatment, which is also associated with considerable cost savings.
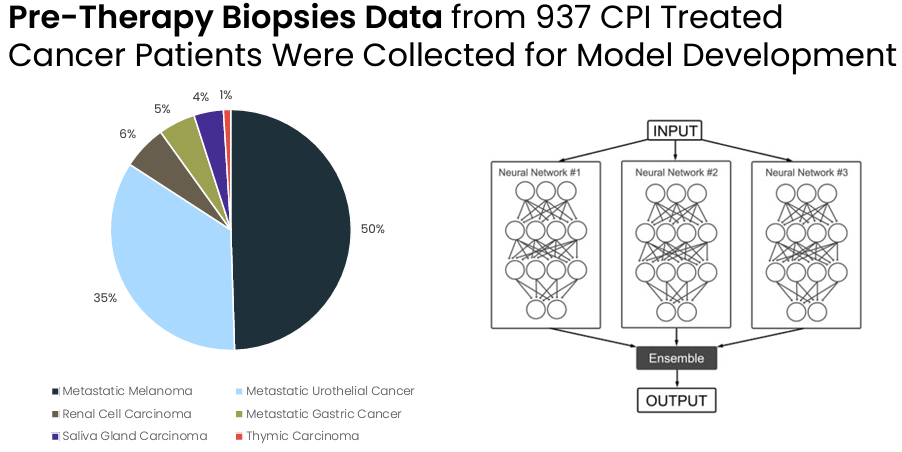
Data based on which the prediction model was developed (Evaxion's R&D day, March 2024)
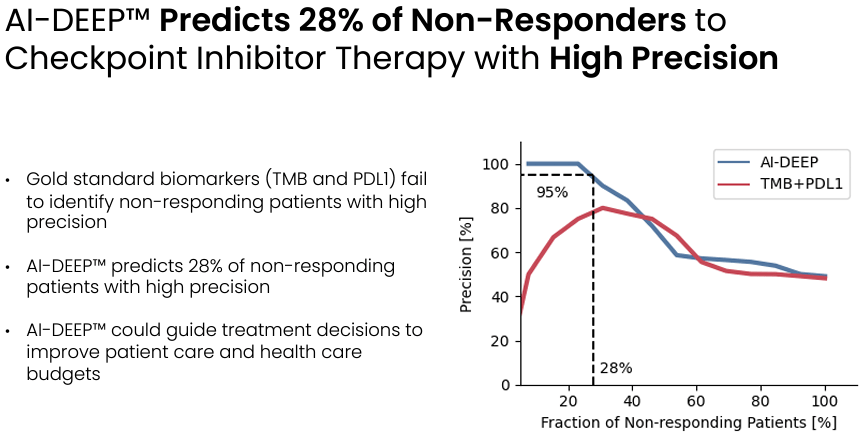
The prediction model can accurately predict about a third of non-responders (Evaxion's R&D day, March 2024)
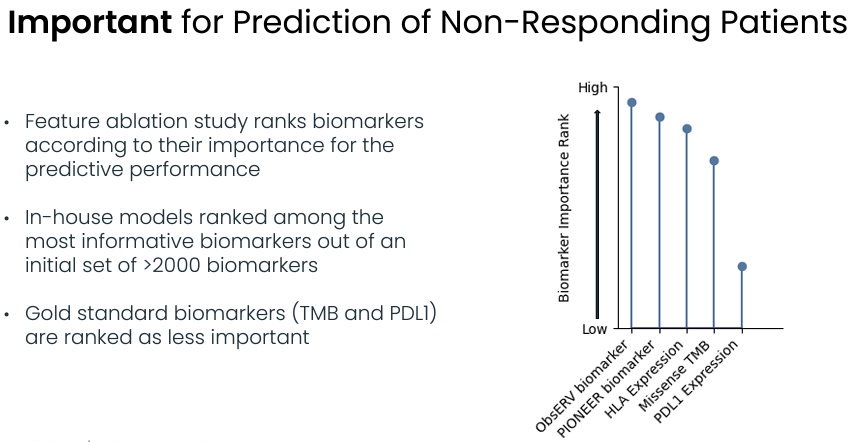
Evaxion's prediction model outperforms alternative biomarkers (Evaxion's R&D day, March 2024)
More POC data are also available from Evaxion's phase 1/2 studies in melanoma, albeit in a small number of patients:
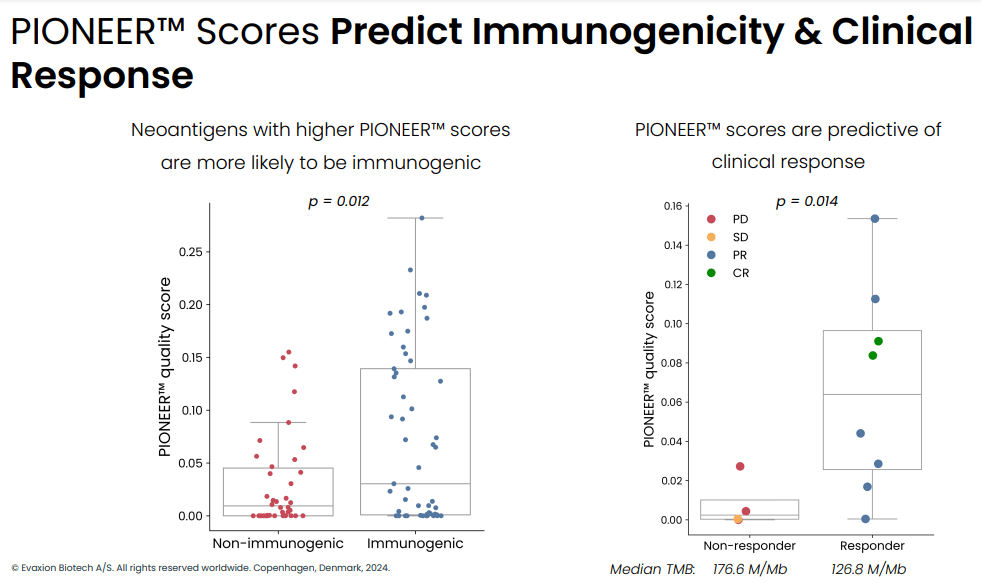
PIONEER score predicts immunogenicity and clinical response (Evaxion R&D day, March 19, 2024)
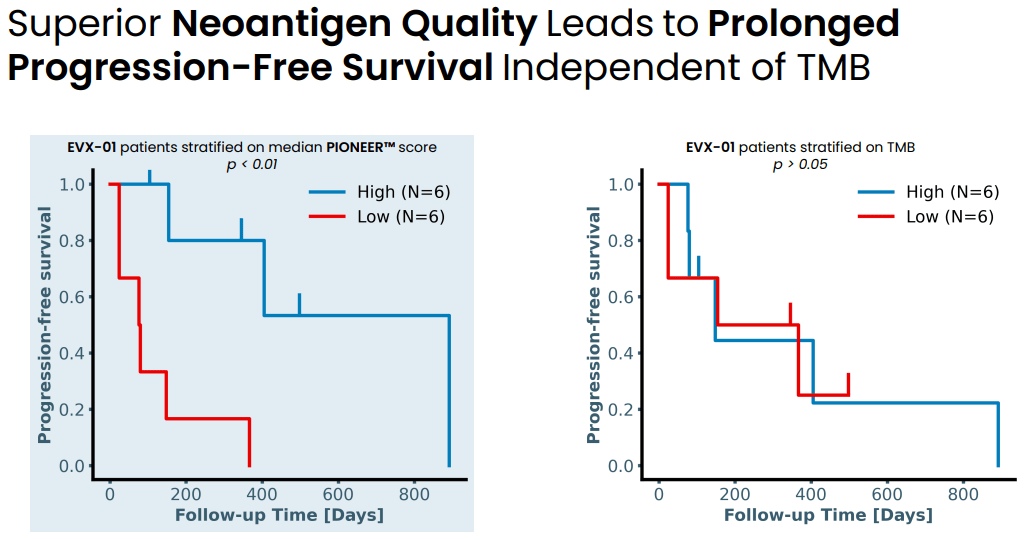
Neoantigen quality based on median PIONEER score predicts response to treatment while tumor mutational burden (TMB) does not (Evaxion's R&D day, March 19, 2024)
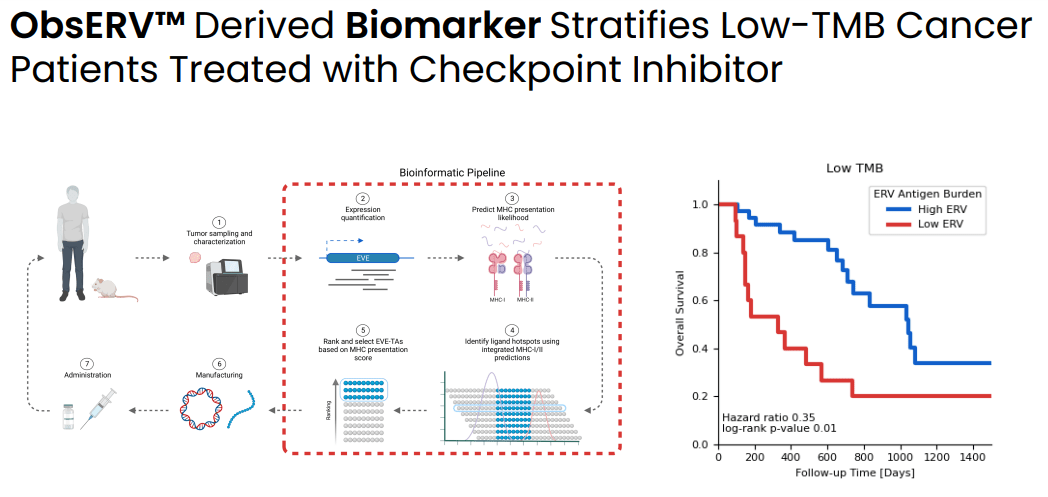
Among patients with low tumor mutational burden ERV antigen burden (as quantified by ObsERV) predicts response to checkpoint inhibitors (Evaxion's R&D day, March 19, 2024)
Evaxion plans to “pursue a partnership-based approach towards a commercial offering” for its AI-DEEP model.
MSD partnership
EVAX has an ongoing collaboration with MSD on two of its assets:
- EVX-01 (discussed above): Under the collaboration agreement MSD is supplying pembrolizumab to be used in combination with EVX-01 for the ongoing multicenter phase 2 study.
- EVX-B3: a vaccine against an undisclosed bacterial pathogen based on EDEN and RAVEN AI models discussed above. By combining both models EVAX aimed to create a vaccine that will elicit both a humoral and cellular immune response. EVAX recently announced successful completion of the initial steps of this collaboration (vaccine target discovery and design phases).
Of interest, MSD has participated in both recent financing rounds and now has an ownership of about 15%. MSD's involvement is promising, demonstrating big pharma interest in EVAX's platform.
Other partnerships
Evaxion announced in September 2023 a partnership with Afrigen Biologics to develop an mRNA vaccine against N. gonorrhea based on Evaxion's EVX-B2 described above. According to the agreement Afrigen will evaluate the activity of the antigens in mRNA format. Following this validation the two companies will negotiate an agreement for clinical development and commercialization, with the opportunity to bring in additional partners. Afrigen will be responsible for clinical development and commercialization in African territories and low- and middle-income countries.
In December 2022 Evaxion had announced a collaboration with ExpreS2ion. EVAX is responsible for designing a vaccine construct based on RAVEN and EDEN. The antigen construct will be produced by ExpreS2ion's ExpreS2 platform to be tested in Evaxion’s state-of-the-art preclinical models. “ExpreS2ion will have the exclusive right to license the CMV vaccine candidate under a potential Development and Commercialization Agreement. The research and intellectual property licensing costs for the collaboration project will be divided 50/50 between the parties until 2025, with all costs expected to be covered by each party’s existing budget. A potential future Development and Commercialisation Agreement for the jointly discovered CMV vaccine candidate is expected to include an upfront payment and future milestone payments to Evaxion from ExpreS2ion not exceeding a six-digit USD amount, as well as sub-licensing royalty to Evaxion from ExpreS2ion based on mid to lower two-digit percentage range of third-party licensee income depending on the clinical development stage of the CMV asset at the time of sublicensing.” The collaboration appears to be ongoing but there doesn't appear to be any news since the December 2022 announcement.
Catalysts in 2024
Many value-creating milestones are anticipated throughout 2024 and are depicted below;
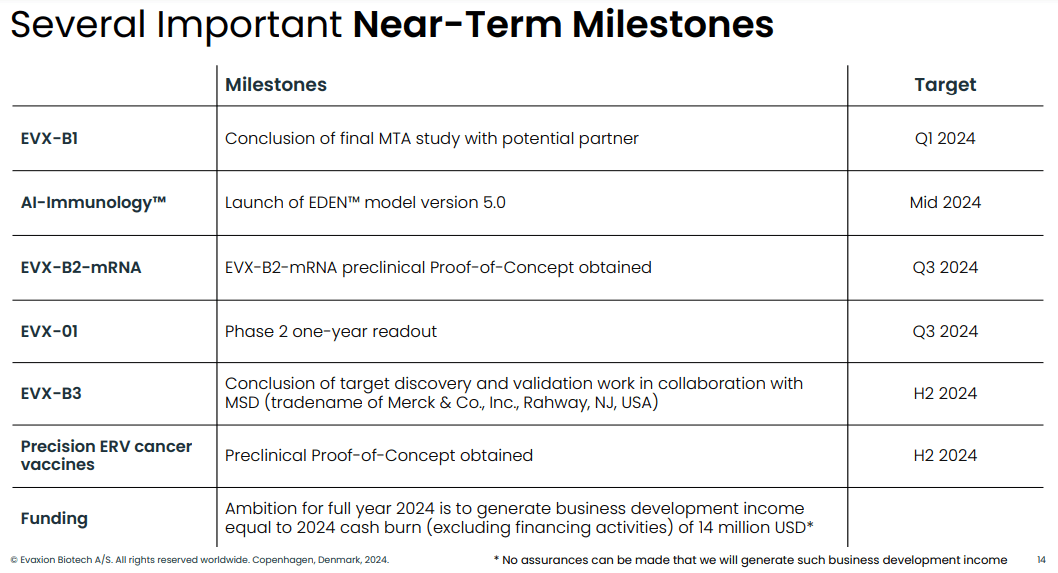
Near-term milestones (Evaxion's R&D day, March 19, 2024)
The most important catalyst would be to achieve non-dilutive funding from existing/new collaborations.
Financials
As of December 2023 EVAX had cash and cash equivalents of $5.6M. Subsequently, EVAX has raised $12.7M (bringing the total to $18.3M). EVAX projects an operational cash burn of $14M for 2024. Based on the above EVAX has guided a cash runway into February 2025, which may be extended into April 2025 if all pre-funded warrants are exercised. EVAX aspires to cover all its 2024 operational cash burn through business transactions (although obviously there is no guarantee this will be achieved). Even with more partnerships I expect EVAX will have to raise more cash soon to support its pipeline. Hopefully, EVAX achieving its goals in 2024 will help boost the stock price and allow financing from a stronger position.
Risks
EVAX's pipeline is still mostly preclinical. So whether the pipeline will ever reach the clinic and whether future assets will be successful and competitive in the clinic remains to be seen. Furthermore, as with any early-stage biotech, the major risk for EVAX is the need to raise a lot more cash to support advancement of the pipeline. Even though EVAX aims to support 2024 operating expenses through business transactions, there is no guarantee this will be achieved. Basing an investment thesis on promises of partnership/non-dilutive funding is rarely a good idea.
Notably, there is immense competition in the field of cancer immunotherapy generally, as well as in the field of personalized cancer vaccines specifically, as summarized in the image below. Whether Evaxion's sophisticated AI Immunology platform will outperform competition remains to be proven. Evaxion's integrated ability to predict which patients will respond could prove to be a huge advantage by allowing enrichment of future clinical trials with patients that have the best chance to respond to treatment.
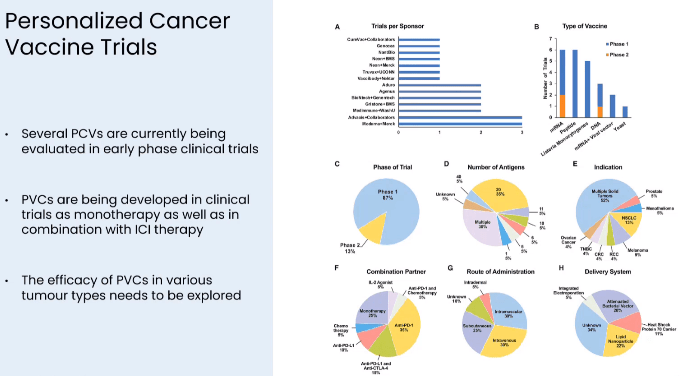
Summary of competition developing personalized cancer vaccines (Presentation of Latest Confirmatory EVX-01 Clinical Data with Key Opinion Leader Adnan Khattak)
Conclusion
EVAX is an intriguing investment. A small speculative position may be worth it considering the following; (1) MSD collaboration and ownership, which validates interest in EVAX's platform by big pharma. (2) POC data in melanoma both for designing an effective vaccine, as well as for predicting which patients will respond to treatment. (3) Preclinical POC data showing that EVAX's EDEN and RAVEN models can quickly and efficiently identify completely novel and highly protective antigens for vaccine design against pathogens for which vaccines are not currently available. (4) Several milestones anticipated throughout 2024. (5) Partnership-based business model (which may allow non-dilutive funding).
On the other hand, EVAX is still a very early-stage biotech which will need a lot more cash. Furthermore, I can't confidently say I am convinced of the uniqueness /advantages of EVAX's platform, maybe due to lack of understanding of the details of its AI technology. So, even though I have personally started a small speculative position, I am giving it a “Hold” recommendation for the time being.
Tip
When investing in penny stocks be prepared to take advantage of spikes in the stock price, which are often very transient and often peak only during pre/after-market hours. A recent example is NCNA, which spiked > +200% from my “Buy” price, but only transiently after-hours following granting of a patent. Therefore, ideally you should be able to trade PM/AH to take advantage of such opportunities.
Your feedback is appreciated
Please comment below if you have any feedback (positive or negative), if you spot any mistakes, or if you believe I missed something important in my analysis.
Also I suggest tracking comments if you are interested in following the stock as I may post updates there.
Editor's Note: This article covers one or more microcap stocks. Please be aware of the risks associated with these stocks.




